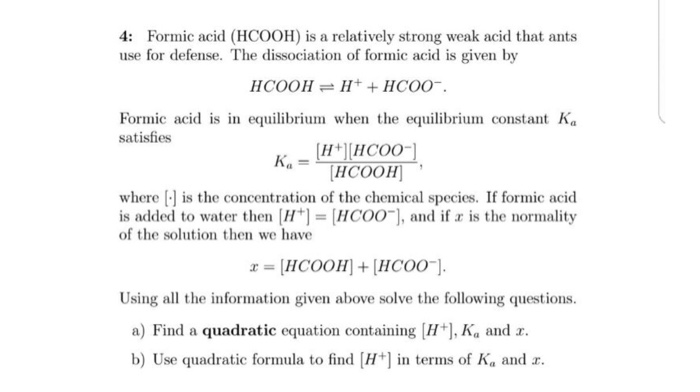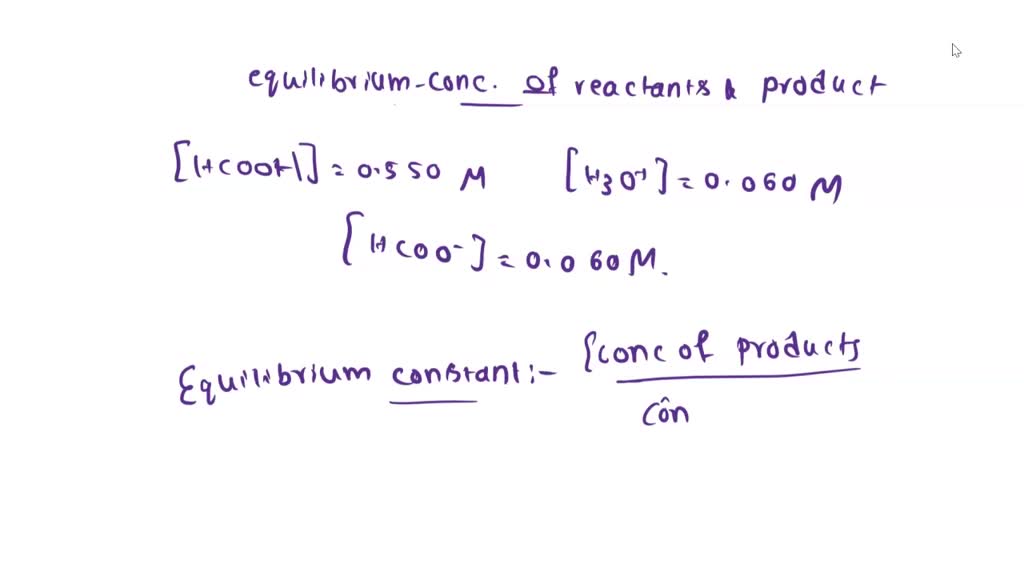
SOLVED: Formic acid dissociates in water according to the equation: HCOOH(aq) HO() H;O (aq) HCOO (aq) Given the following equilibrium concentrations; calculate the equilibrium constant: IHCOOHI = 0.550 M, [o [ =

Acids at the Edge: Why Nitric and Formic Acid Dissociations at Air–Water Interfaces Depend on Depth and on Interface Specific Area | Journal of the American Chemical Society

In acid buffer solution (pH = 4.4), the ratio of concentrations of acid to salt is 2 : 1. The value of dissociation constant of weak acid may be:
![Weak Acids & Bases Chapter 16. Dissociation Constants Since weak acids do not dissociate completely, [H 3 O + ] ≠ [acid] For a generalized acid dissociation, - ppt download Weak Acids & Bases Chapter 16. Dissociation Constants Since weak acids do not dissociate completely, [H 3 O + ] ≠ [acid] For a generalized acid dissociation, - ppt download](https://images.slideplayer.com/33/9517443/slides/slide_4.jpg)
Weak Acids & Bases Chapter 16. Dissociation Constants Since weak acids do not dissociate completely, [H 3 O + ] ≠ [acid] For a generalized acid dissociation, - ppt download
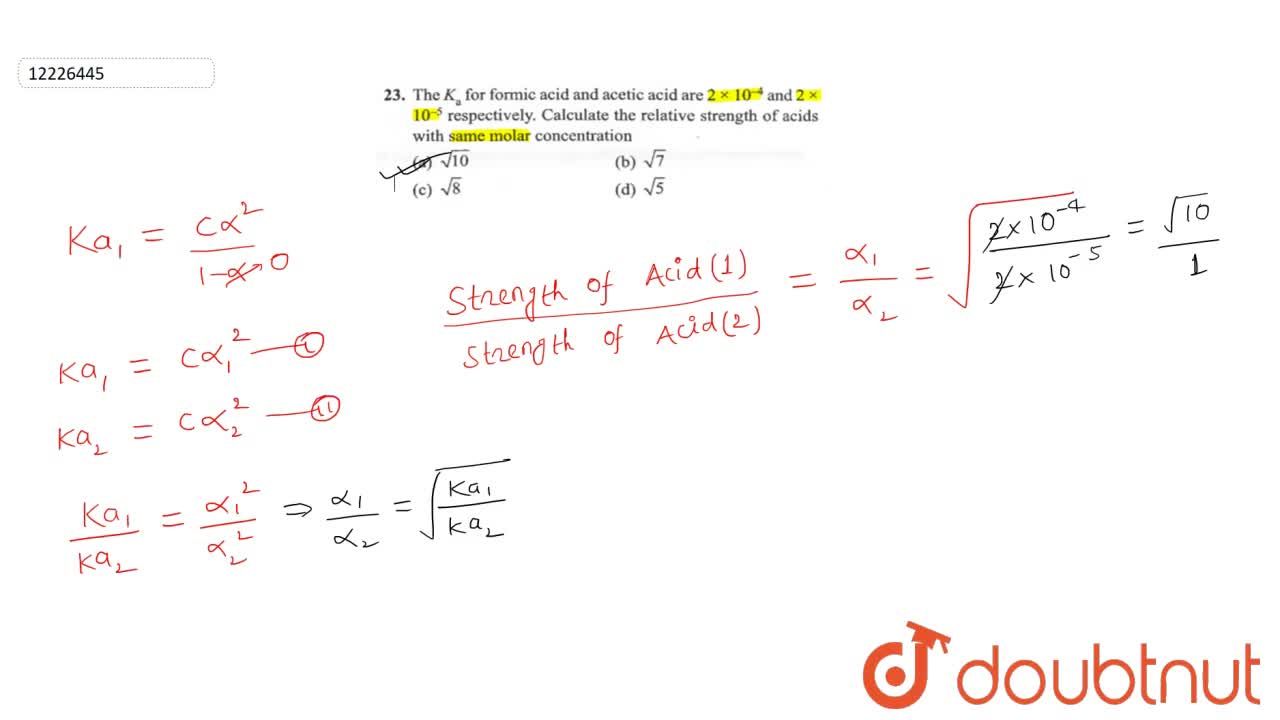
The K(a) for formic acid and acetic acid are 2xx10^(-4) and 2xx10^(-5) respectively. Calculate the relative strength of acids with same molar concentration

Dissociation constant (Ka) of formic acid and acetic acid are 2.5xx10^-4 and 0.5xx10^-5 respectively. The ratio of their relative strengths at the same concentration is

Complex Mechanism of the Gas Phase Reaction between Formic Acid and Hydroxyl Radical. Proton Coupled Electron Transfer versus Radical Hydrogen Abstraction Mechanisms | Journal of the American Chemical Society
![The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The](https://dwes9vv9u0550.cloudfront.net/images/11469763/0c5bdcb3-4186-4faf-92ed-af469406afbf.jpg)
The self ionization constant for pure formic acid, K = [HCOOH^-2] [HCOO^ - ] has been estimated as 10^-6 at room temperature. The density of formic acid is 1.22 g/cm^3 . The

The dissociation constants of formic and acetic acids are 1.77 × 10^-4 and 1.75 × 10^-5 , respectively
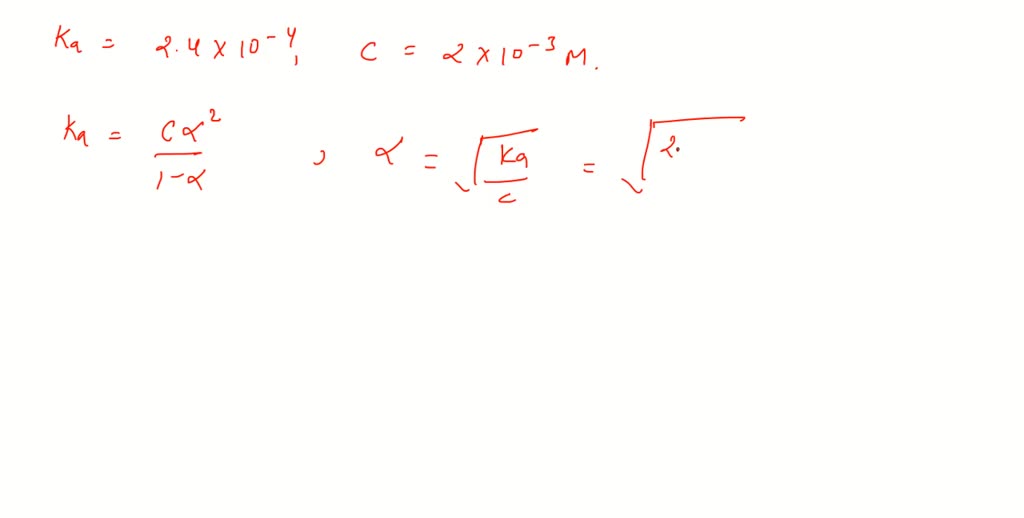
SOLVED:The dissociation constant of formic acid is 0.00024. The hydrogen ion concentration in 0.002 M-HCOOH solution is nearly (a) 6.93 ×10^-4 M (b) 4.8 ×10^-7 M (c) 5.8 ×10^-4 M (d) 1.4 ×10^-4 M
The self-ionization constant for pure formic acid, K = [HCOOH2+] [HCOO–] has been estimated as 10–6 at room temperature. What percentage of formic acid molecules in pure formic acid are converted to

What is the pH of a 0.0944 M aqueous solution of formic acid, HCOOH? (Ka = 1.8 x 10-4) | Homework.Study.com
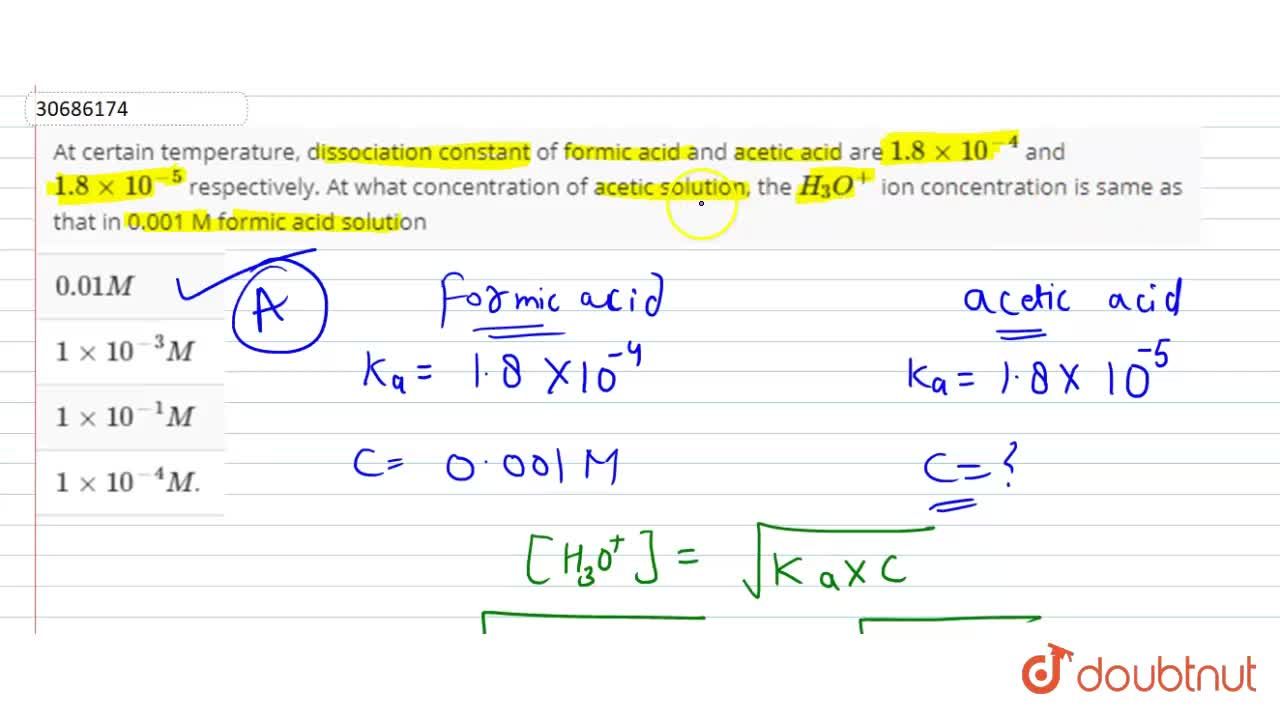
At certain temperature, dissociation constant of formic acid and acetic acid are 1.8xx10^(-4) and 1.8xx10^(-5) respectively. At what concentration of acetic solution, the H93)O^(+) ion concentration is same as that in 0.001