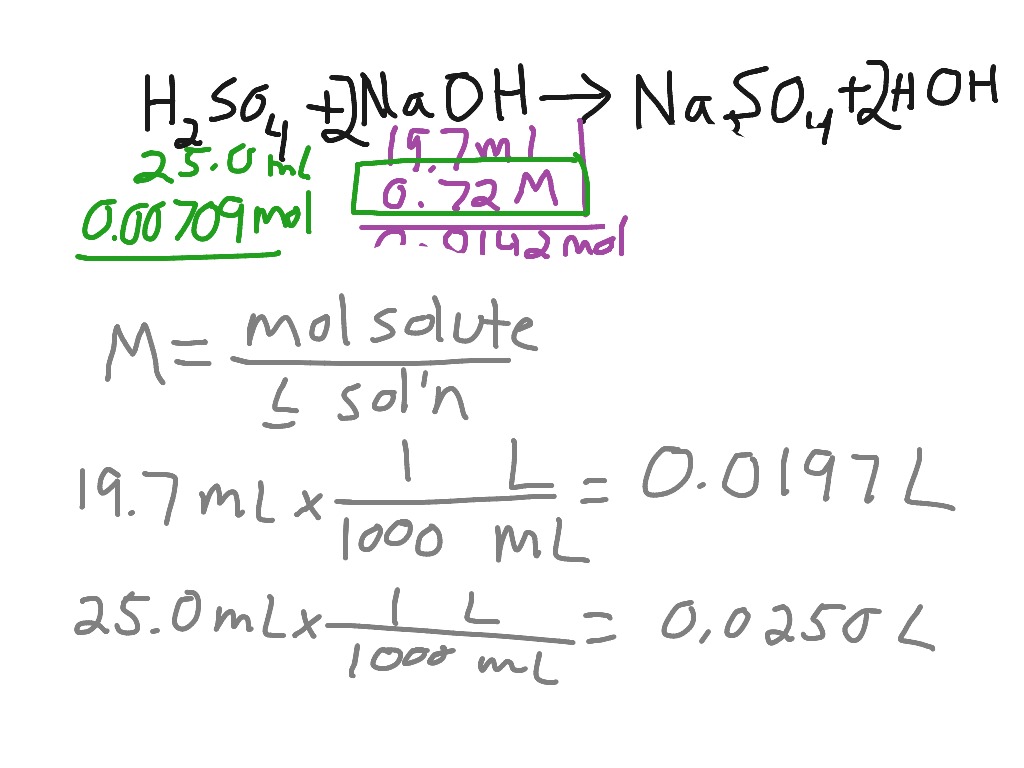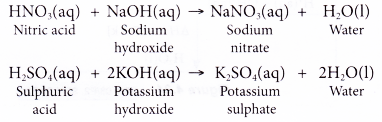WARM UP 1. Write the equation for the neutralization reaction between sulfuric acid (H 2 SO 4 ) and ammonium hydroxide (NH 4 OH). - ppt download

SOLVED: Suppose you want to neutralize a sulfuric acid spill. Calculate the (minimum) grams of sodium bicarbonate (NaHCO3) required to neutralize 1.8 mL of 2.25 M sulfuric acid according to the following
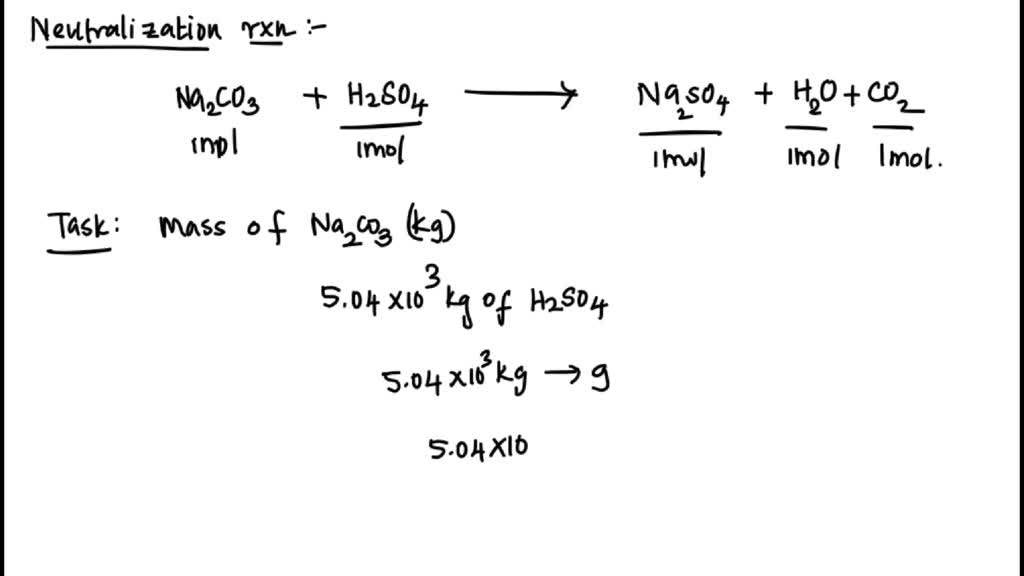
SOLVED: Sodium carbonate (Na2CO3Na2CO3) is used to neutralize the sulfuric acid spill. How many kilograms of sodium carbonate must be added to neutralize 5.04×103 kgkg of sulfuric acid solution?

SOLVED: Assignment 3 In a titration of sulfuric acid against sodium hydroxide, 32.20mL of0.250 M NaOH is required to neutralize 26.60mL of H,SOA Calculate the molarity of the sulfuric acid. E,SO, (aq) +

SOLVED: Determine the volume of 0.240 M KOH solution required to neutralize each sample of sulfuric acid. The neutralization reaction is: H2SO4(aq)+2KOH(aq)→ K2SO4(aq)+2H2O(l) 40 mLmL of 0.240 MM H2SO4H2SO4 Express your answer
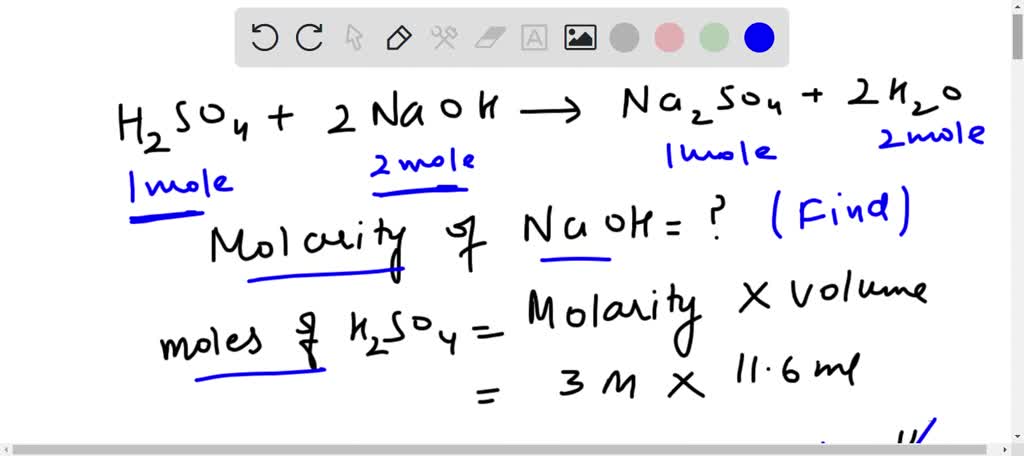
SOLVED: Calculating Molarity from Titration data reveals that 11.6 mL of 3.0 M sulfuric acid are required to neutralize the sodium hydroxide in 25.00 mL of NaOH solution. What is the molarity

What volume of 0.147 molar sulfuric acid solution would be required to neutralize completely 36 ml of - Brainly.com