Determination of concentration of KMnO₄ solution (Theory) : Class 12 : Chemistry : Online Labs for schools - Developed by Amrita Vishwa Vidyapeetham and CDAC Online Lab
Which is the type of reaction of potassium permanganate and oxalic acid: endothermic or exothermic? - Quora

KMnO(4) react with oxalic acid according to the equation, 2MnO(4)^(-)+5C(2)O(4)^(2-)+16H^(+) rarr 2Mn^(2+)+ 10 CO(2)+8H(2)O, here 20 ml of 0.1 M KMnO(4) is equivalemt to

To determine the Molarity of KMnO4 solution by titrating it against a standard solution of oxalic acid. (M/20 oxalic acid solution).
The Kinetics of the Reaction between Potassium Permanganate and Oxalic Acid. II | Journal of the American Chemical Society

science chemistry titration potassium permanganate sodium dichromate | Fundamental Photographs - The Art of Science

314. The reaction between potassium permanganate and oxalic acid - Journal of the Chemical Society (Resumed) (RSC Publishing)

Standard oxalic acid concentration vs. burette readings i.e. ml of KMnO... | Download Scientific Diagram
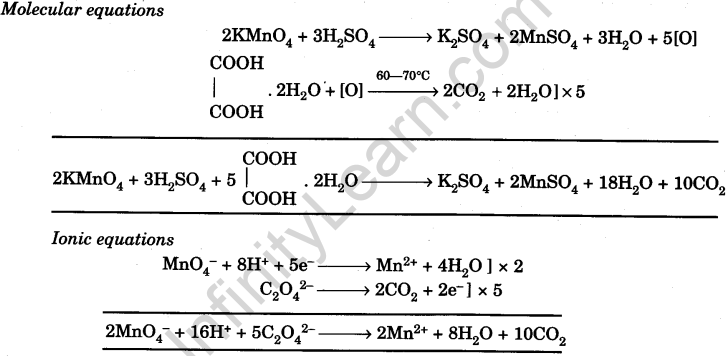
Prepare N/20 solution of oxalic acid. Using this solution, find out strength and normality of the given potassium permanganate solution

When potassium permanganate, `KMnO_(4)` , is added to an acidified solution of oxalic acid, - YouTube

KMnO4 reacts with oxalic acid according to the equation, 2MnO ^ - 4 + 5C2O^2 - 4 + 16H^ + → 2Mn^2 + + 10CO2 + 8H2O , here 20 ml of 0.1 M KMnO4 is equivalent to:

Explain why does color of KMnO4 disappear when oxalic acid is added to its solution in acidic medium.