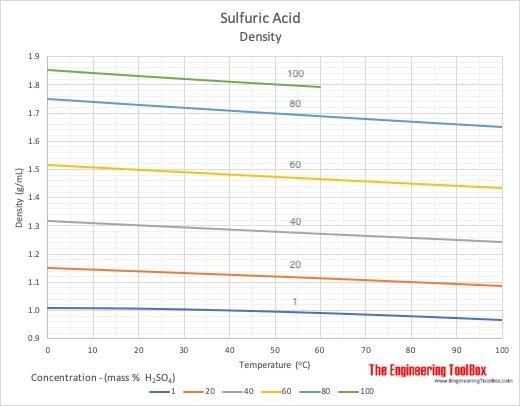
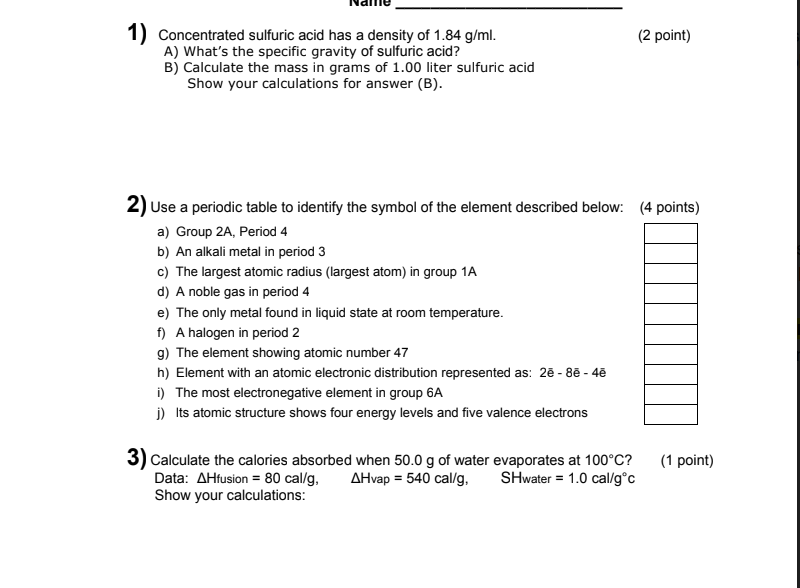
28. concentrated sulphuric acid has a density of 1.9 g/ml and is 99 percent h2so4 by weight calculate molarity of h2so4 in this acid

Laboratory grade concentrated sulphuric acid has a density 1.82g cc^(-1) .Weight percentage of acid is 98. Calculate the normality of solution.
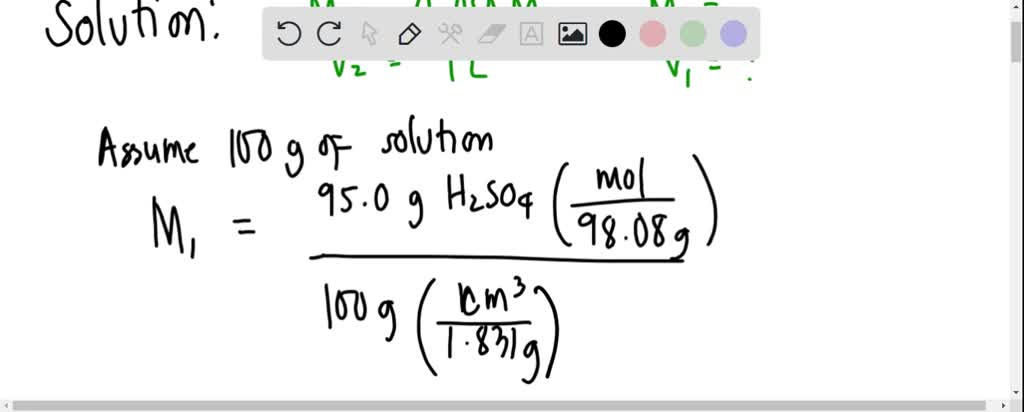
SOLVED: Calculate the volume (in millilitres) of concentrated sulfuric acid, 95.0% (g/100 solution) with a density of 1.831 g/cm3 required to prepare 1 L of a 0.050 M solution.

Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 gm L^-1 . What is the volume of acid required to make one litre of 0.1 M H2SO4 solution?

Concentrated sulphuric acid has density of 1.9 g/mL and 99% H2SO4 by mass. Calculate the molarity of the acid.

a bottle of concentrated sulphuric acid (density of 1.80 g cm-3) is labelled as 86% as weight. What is - Brainly.in

Concentrated H2SO4 has a density 1.9g/ml and is 99% H2SO4 by mass. Calculate the molarity. - YouTube
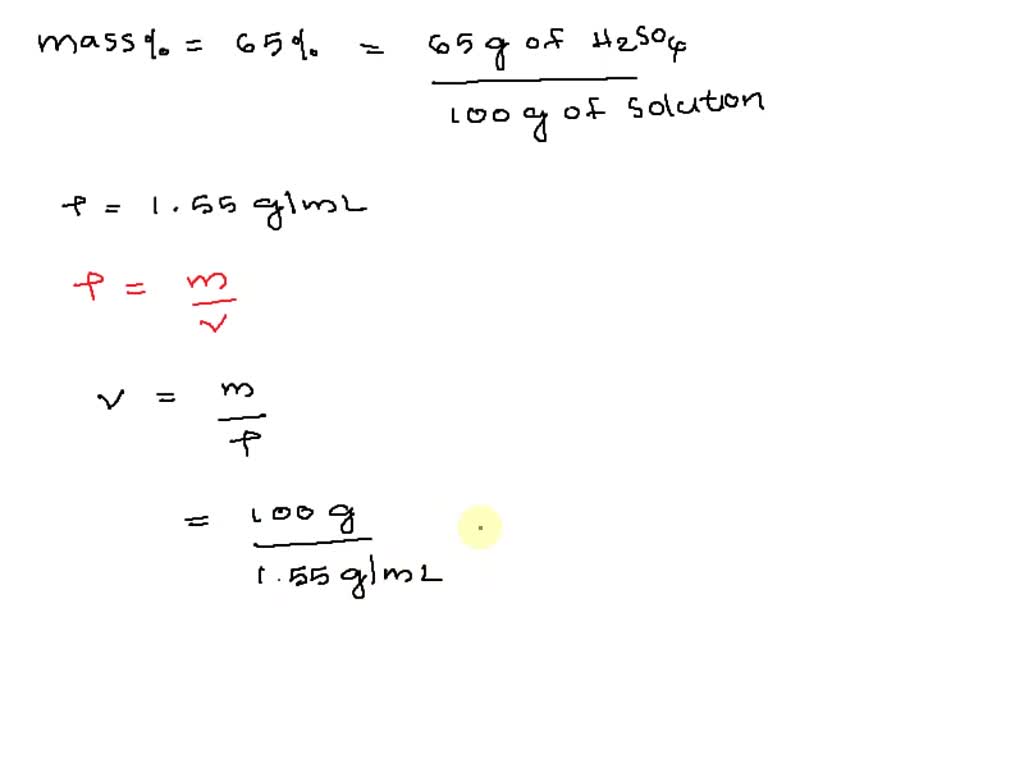
SOLVED: A concentrated sulfuric acid solution is 65.0% H2SO4 by mass and has a density of 1.55 g/mL at 20°C. What is the mass of 9.00 L of the concentrated sulfuric acid
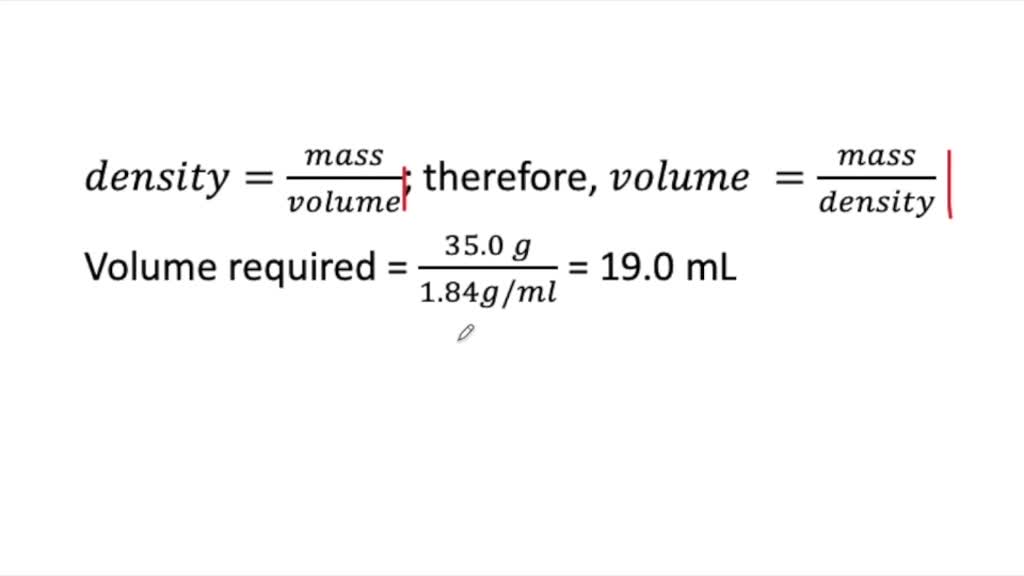
SOLVED:A chemist needs 35.0 g of concentrated sulfuric acid for an experiment. The density of concentrated sulfuric acid at room temperature is 1.84 g / mL. What volume of the acid is required?

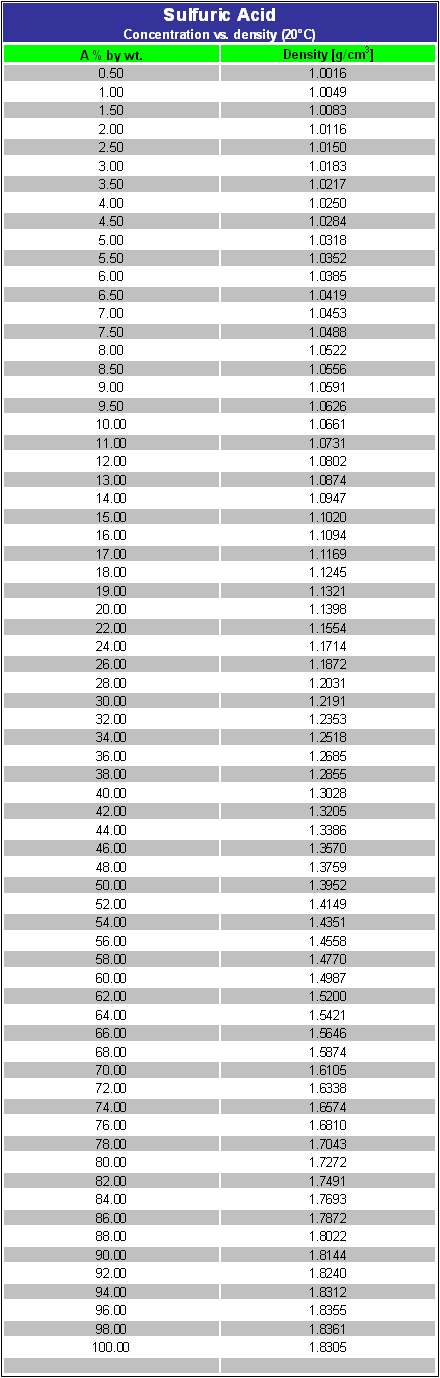
![ANSWERED] Concentrated sulfuric acid (18.3 M) has ... - Inorganic Chemistry ANSWERED] Concentrated sulfuric acid (18.3 M) has ... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/56261425-1660029604.3224282.jpeg)