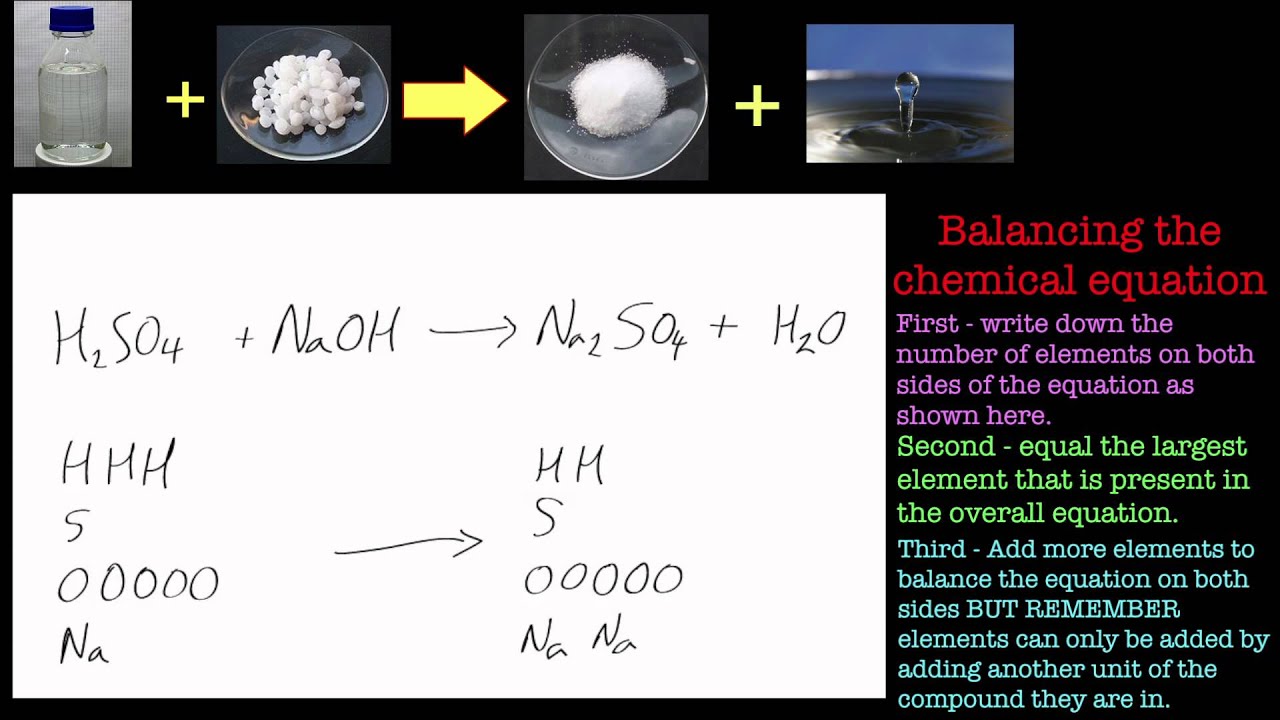
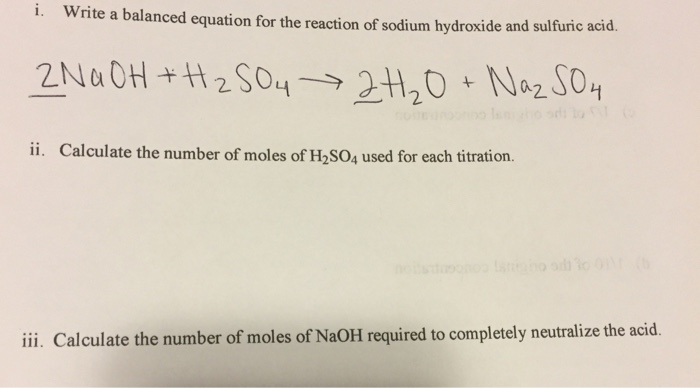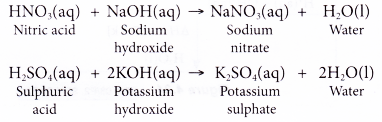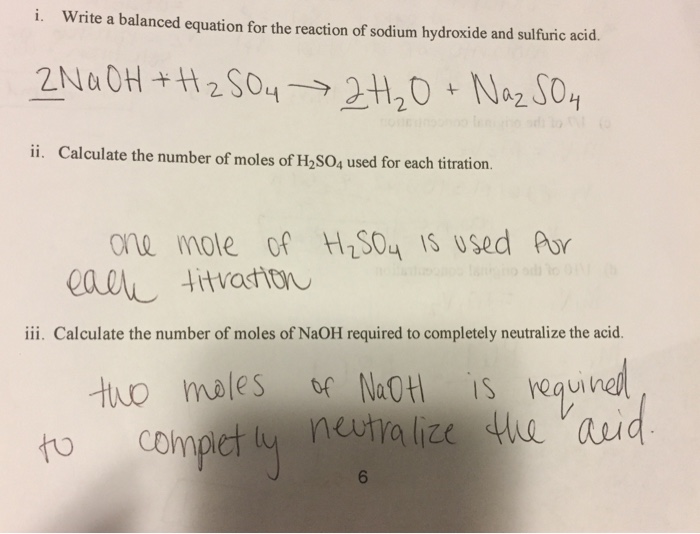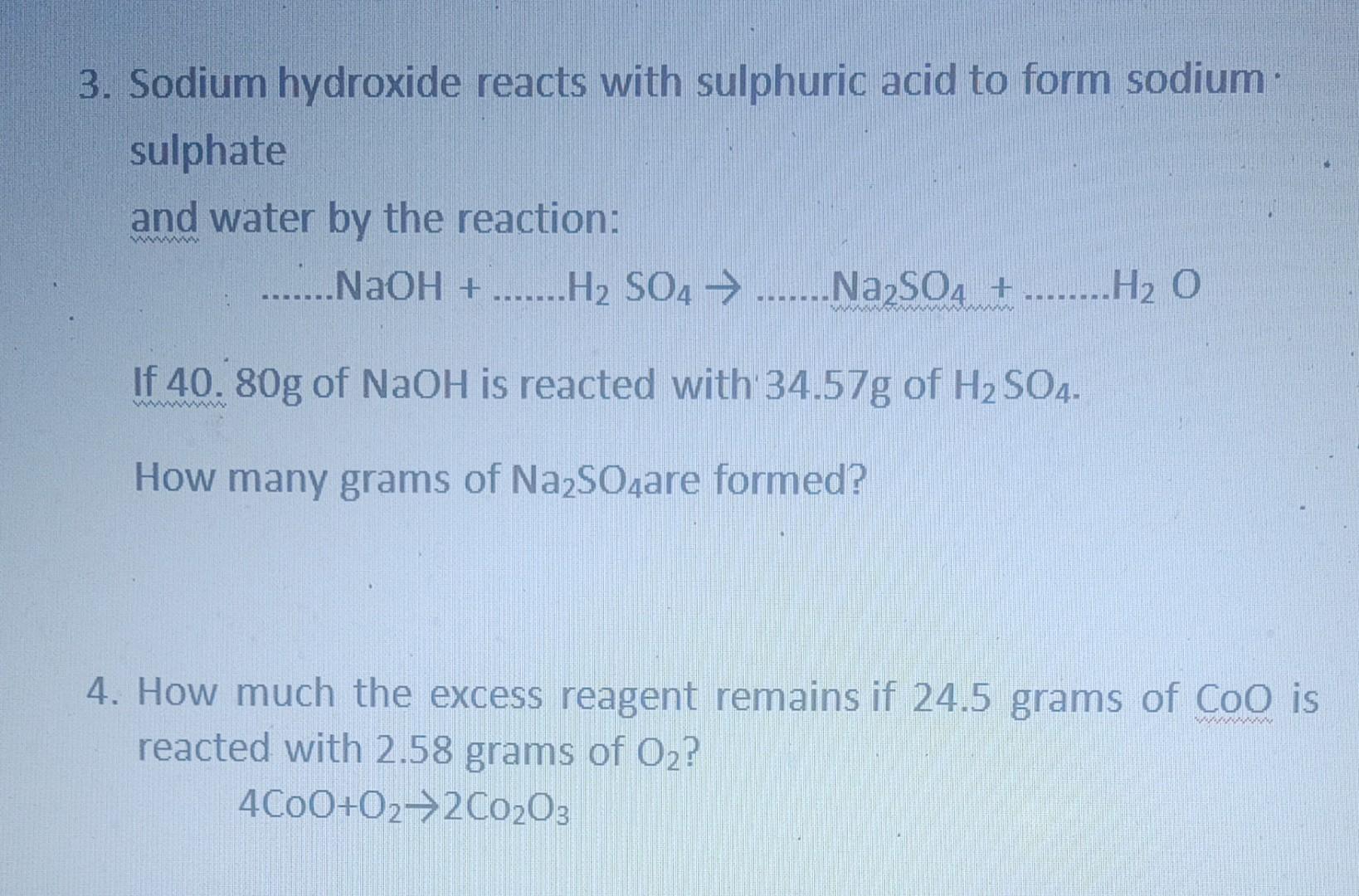Sulphuric acid reacts with sodium hydroxide as follows H2SO4 + 2NaOH → Na2SO4 + 2H2O When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of

SOLVED:Challenge Sulfuric acid (H2 SOA) and sodium hydroxide solutions react to produce aqueous sodium sulfate and water.
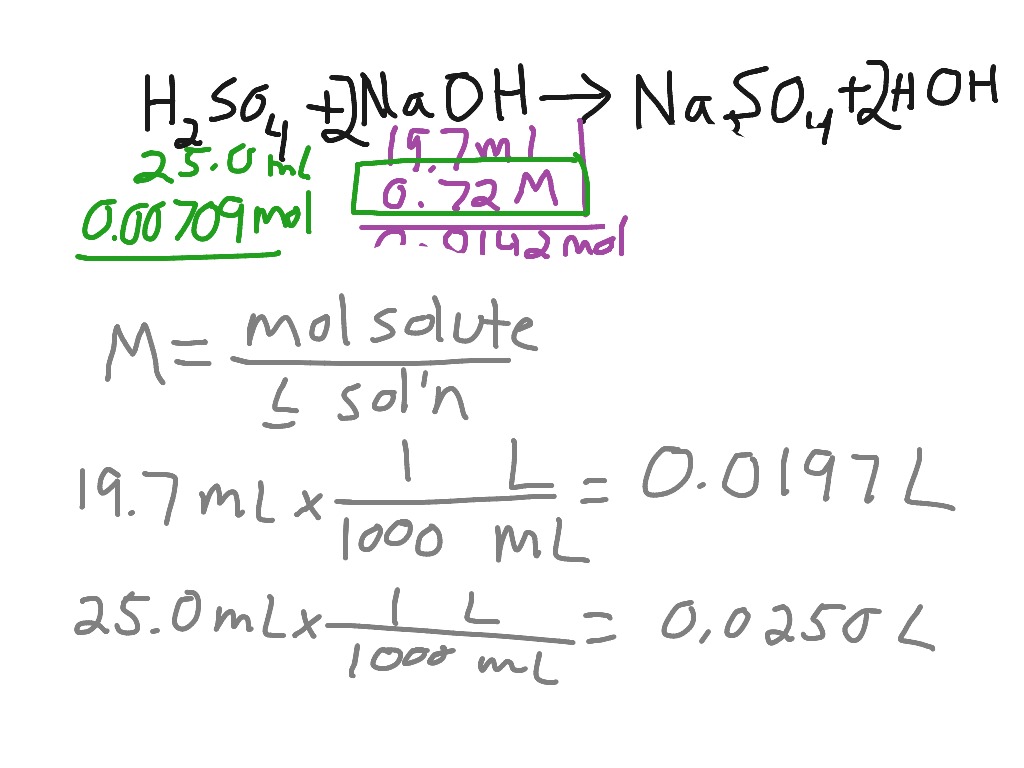
Titration of sulfuric acid with sodium hydroxide | Chemistry, Acids and Bases, Stoichiometry | ShowMe

SOLVED:Sulfuric acid reacts with sodium hydroxide according to the following: H2 SO4+NaOH ⟶Na2 SO4+H2 O a. Balance the equation for this reaction. b. What mass of H2 SO4 would be required to
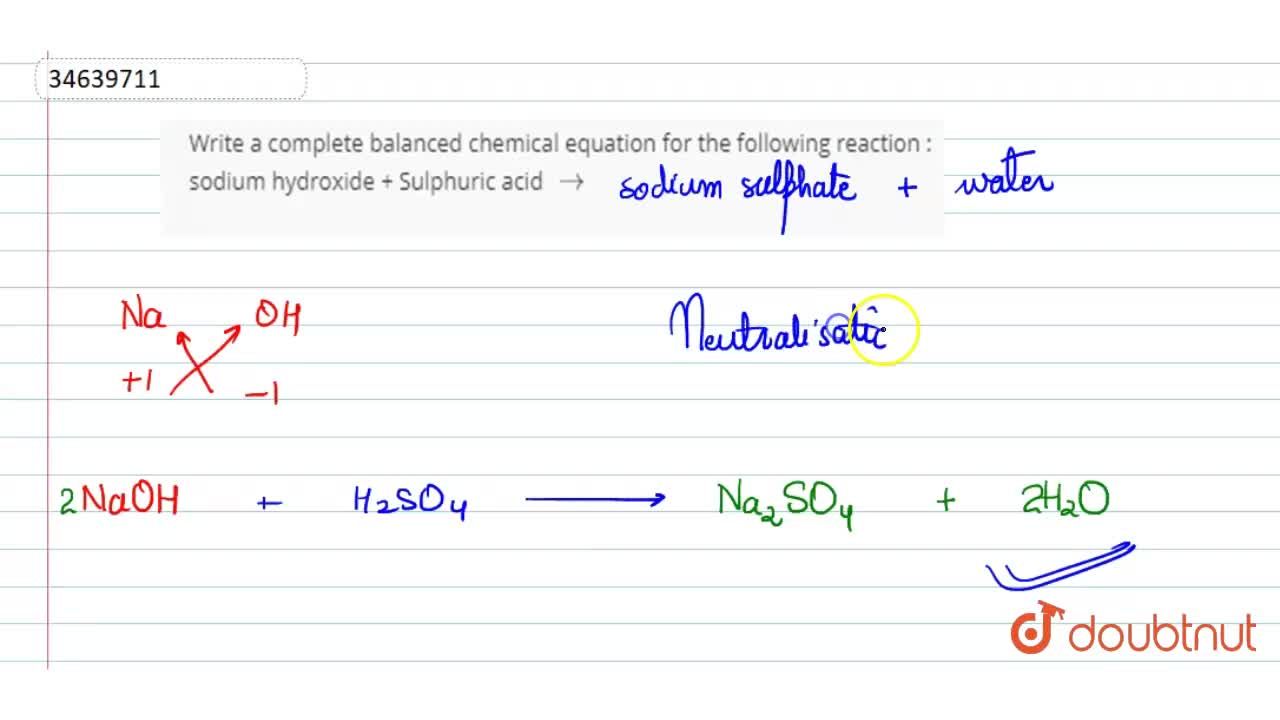
Write a complete balanced chemical equation for the following reaction : sodium hydroxide + Sulphuric acid to
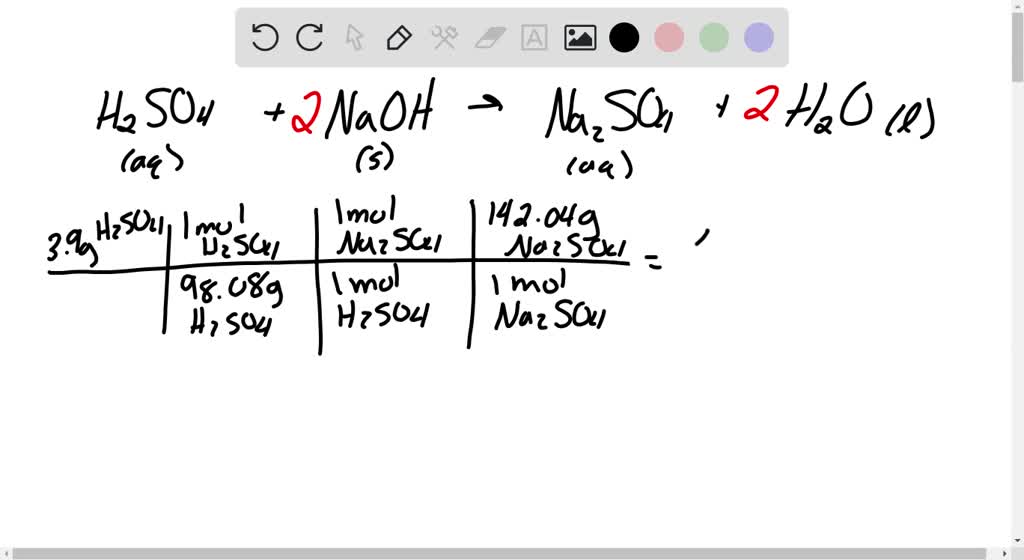
SOLVED: Aqueous sulfuric acid (H2SO4) reacts with solid sodium hydroxide ( NaOH) to produce aqueous sodium sulfate (Na2SO4) and liquid water (H2O). If 1.58 g of sodium sulfate is produced from the reaction

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download

Sulphuric acid reacts with sodium hydroxide as follows H(2)SO(4)+2NaOHrarrNa(2)SO(4)+2H(2)O when 1L of 0.1M sulphuric acid solution is allowed to react with 1L of 0.1M sodium hydroxide solution, the amount of sodium solphate

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

2.46 g of sodium hydroxide (molar mass = 40) are dissolved in water and the solution is made to 100 cm ^3 in a volumetric flask. Calculate the molarity (in mol/L) of the solution.